Standard Reduction Potential Gold . the reference state for amalgams is an infinitely dilute solution of the element in hg. The following table provides eo for selected reduction. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the standard reduction potential is measured under standard conditions: the following table provides e o and e o ´ values for selected reduction reactions. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Standard reduction potentials by value. T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for. The temperature coefficient, de /dt allows us to calculate.
from www.chegg.com
assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. The following table provides eo for selected reduction. the standard reduction potential is measured under standard conditions: the following table provides e o and e o ´ values for selected reduction reactions. T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. the reference state for amalgams is an infinitely dilute solution of the element in hg. Standard reduction potentials by value. The temperature coefficient, de /dt allows us to calculate.
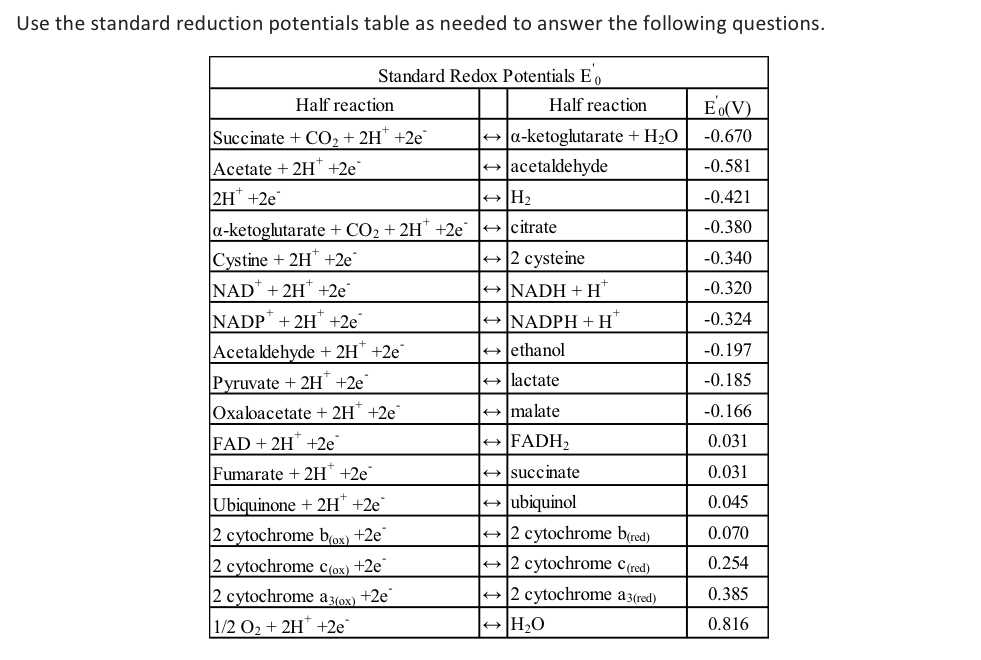
Solved Use the standard reduction potentials table as needed
Standard Reduction Potential Gold The following table provides eo for selected reduction. The temperature coefficient, de /dt allows us to calculate. Standard reduction potentials by value. The following table provides eo for selected reduction. the following table provides e o and e o ´ values for selected reduction reactions. the standard reduction potential is measured under standard conditions: the reference state for amalgams is an infinitely dilute solution of the element in hg. T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Gold the standard reduction potential is measured under standard conditions: the following table provides e o and e o ´ values for selected reduction reactions. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the values of standard electrode potentials are given in the table in volts relative to. Standard Reduction Potential Gold.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials Chemistry Standard Reduction Potential Gold assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Standard reduction potentials by value. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. 372 rows the data below tabulates standard electrode potentials (e °), in volts. Standard Reduction Potential Gold.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Gold the standard reduction potential is measured under standard conditions: The temperature coefficient, de /dt allows us to calculate. the reference state for amalgams is an infinitely dilute solution of the element in hg. The following table provides eo for selected reduction. Standard reduction potentials by value. 372 rows the data below tabulates standard electrode potentials (e °),. Standard Reduction Potential Gold.
From www.chegg.com
Solved Use standard reduction potentials to calculate the Standard Reduction Potential Gold 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the following table provides e o and e o ´ values for selected reduction reactions. The temperature coefficient, de /dt allows us to calculate. T = 298.15 k (25 °c, or 77 °f), a unity activity (a. Standard Reduction Potential Gold.
From hasyimmi.blogspot.com
Standard Reduction Potential Table Reduction Potentials in the Standard Reduction Potential Gold 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the standard reduction potential is measured under standard conditions: assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the following table provides e o and e o. Standard Reduction Potential Gold.
From www.researchgate.net
CV and redox potential for the Fe(III)/Fe(II) redox couple of different Standard Reduction Potential Gold Standard reduction potentials by value. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The temperature coefficient, de /dt allows us to calculate. the standard reduction potential is measured under standard conditions: the following table provides e o and e o ´ values for selected. Standard Reduction Potential Gold.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Learning Inc Standard Reduction Potential Gold the reference state for amalgams is an infinitely dilute solution of the element in hg. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. the following table provides e o and e o ´ values for selected reduction reactions. T = 298.15 k (25. Standard Reduction Potential Gold.
From www.pinterest.com
Standard Reduction Potential (E) when given two half reactions and Standard Reduction Potential Gold the standard reduction potential is measured under standard conditions: 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The temperature coefficient, de /dt allows us to calculate. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. . Standard Reduction Potential Gold.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Gold the standard reduction potential is measured under standard conditions: 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the following table provides e o and e o ´ values for selected reduction reactions. T = 298.15 k (25 °c, or 77 °f), a unity activity. Standard Reduction Potential Gold.
From www.flinnsci.com
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Gold assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The following table provides eo for selected reduction. The temperature coefficient, de /dt allows us to calculate. the following. Standard Reduction Potential Gold.
From boisestate.pressbooks.pub
17.3 Standard Reduction Potentials General Chemistry 1 & 2 Standard Reduction Potential Gold the standard reduction potential is measured under standard conditions: the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. The following table provides eo for selected reduction. the following table provides e o and e o ´ values for selected reduction reactions. Standard reduction potentials. Standard Reduction Potential Gold.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Gold The following table provides eo for selected reduction. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Standard reduction potentials by value. 372 rows the data below. Standard Reduction Potential Gold.
From www.slideserve.com
PPT ELECTROCHEMISTRY Chapter 21 PowerPoint Presentation, free Standard Reduction Potential Gold assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen. Standard Reduction Potential Gold.
From ch302.cm.utexas.edu
Electrochemistry_Reduction_Potentials Standard Reduction Potential Gold assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. Standard reduction potentials by value. The temperature coefficient, de /dt allows us to calculate. The following table provides eo for selected reduction. the following table provides e o and e o ´ values for selected reduction reactions. the standard reduction. Standard Reduction Potential Gold.
From labbyag.es
Reduction Potential Chart Labb by AG Standard Reduction Potential Gold the following table provides e o and e o ´ values for selected reduction reactions. the standard reduction potential is measured under standard conditions: The following table provides eo for selected reduction. the reference state for amalgams is an infinitely dilute solution of the element in hg. The temperature coefficient, de /dt allows us to calculate. . Standard Reduction Potential Gold.
From www.youtube.com
The diagonal rule of the standard reduction potential table (Part II Standard Reduction Potential Gold T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for. The temperature coefficient, de /dt allows us to calculate. The following table provides eo for selected reduction. the standard reduction potential is measured under standard conditions: the reference state for amalgams is an infinitely dilute solution of the element in hg. . Standard Reduction Potential Gold.
From www.solutioninn.com
[Solved] Use the table of standard reduction poten SolutionInn Standard Reduction Potential Gold T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. The following table provides eo for selected reduction. 372 rows the data below tabulates standard electrode potentials (e °), in. Standard Reduction Potential Gold.
From www.scribd.com
Standard Reduction Potentials Data Extended pdf Standard Reduction Potential Gold 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. The temperature coefficient, de /dt allows us to calculate. The following table provides eo for. Standard Reduction Potential Gold.